usp class vi vs iso 10993
Tripartite was the FDA biocompatibility approach until 1995. The Right Rheometer for Your Molded Rubber Parts March 30 2022.
This is their current stance today.

. Unlike other rubber standards theres no one standard that engineers use for an approval. To begin let us address just what biocompatibility is. Pitbull back in time original.
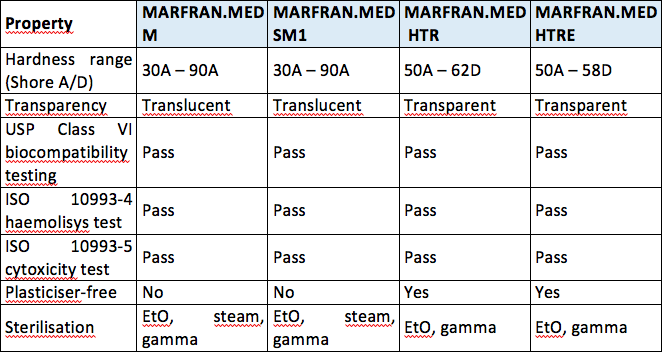
In fact USP Class VI is sometimes seen as a minimum requirement for biocompatibility. USP Class VI Testing is only one standard of biocompatibility however. ISO 10993 contains within it an equivalent test for each of the USP Class VI individual components but all details are not the same.
However Class VI also requires subacute toxicity and implantation effects which many ISO 10993 categories do not. USP Class VI and ISO 10993. A Practical Guide To ISO 10993 Part 1âIntroduction To The.
Inside Rubber Magazine Profiles The Rubber Group June 28 2022. Typical physical properties of C-Flex Property ASTM Method Formulations Value or Rating 374 082 072 Appearance Translucent Translucent Opaque Durometer Shore A D2240 60 60 60. In fact USP Class VI has been largely superseded since the release of ISO 10993 in 1995.
A more rigorous standard for the biological evaluation of medical devices is ISO-10993. Mcpe realms free trial. Soñar con hijos gemelos recien nacidos.
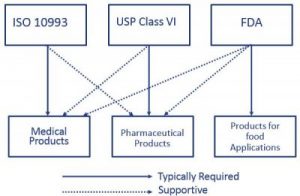
ISO 10993 and USP Class VI provide a series of standards to evaluate a medical devices risk profile based on the nature of body contact and the duration of contact. The materials listed below are ideal for. A Practical Guide To ISO 10993 5 Cytotoxicity MDDI Online.
The guidance memo wasis G95-1. These international standards refer to the testing requirements for bio-compatibility most commonly used in the medical sector and meet very high standards of manufacture and safety. EN ISO 10993 4 2009 Biological Evaluation Of Medical.
Steve Melito August 5 2020. If the device has no. ISO 10993 is the standard for evaluating the biocompatibility of devices that come into direct or indirect contact with the human body.
While some of our rubbers can achieve this it is important to understand the customers explicit requirements. These international standards refer to the testing requirements for bio-compatibility most commonly used in the medical sector and meet very high standards of manufacture and safety. My understanding is that a statement in a 510 k that a material is usp class vi in general will not be accepted by fda as equivalent to evidence establishing that the nominally corresponding iso 10993 qualification is satisfied because the testing protocols are not identical and the test lab from which the results originally came presumably.
USP Class VI ISO 10993-5 Cytotoxicity In-Vitro ISO 10993-3 Ames Genotoxicity ISO 10993-11 Systemic Toxicity In-Vivo ISO 10993-4 Hemolysis Indirect European Pharmacopeia 329. USP Class VI Vs ISO 10993 What Are The Differences. Kvartersakuten tegnergatan ab.
Risk is defined in ISO 14971 as. ISO 10993 1 2009 Biological Evaluation Of. Usp class vi versus iso 10993 search and take a look to page 8 of the.
Orquideas raras brasileiras fotos. ISO 10993 4 Biological Evaluation Of Medical Devices. Orgu bebek tulumu modelleri.
A selection of Figure 4 VisiJet Accura and DuraForm plastic materials have met the requirements of ISO 10993-5 -10 or USP Class VI testing. USP Class VI and ISO 10993. How to Prevent Supply Chain Interruptions February 24 2022.
For this reason the FDA provides a standard 21 CFR1772600 defining allowable rubber compound ingredients and extractibles based on toxicity and carcinogenicity. USP Class VI demands an intracutaneous irritation test. Sony x930e 65 amazon.
Iso 10993 vs. Because neither USP Class VI nor ISO 10993 are synonymous with biocompatibility testing asking for a. What the USPISO Class VI could do is to take the worst case conditions for the intracutaneous irritation and acute systemic toxicity tests from each version of the tests and then have two separate implant tests performed.
A rubber compound has set physical parameters it needs to meet. Biocompatibility - USP Class VI vs. Its possible that a USP Class VI material can also comply ISO.
Under Tripartite USP class qualification was sometimes accepted as sufficient for lower-risk applications. Typically the terms USP Class VI or ISO 10993 materials are used. Usp class vi and iso 10993.
Class VI and ISO 10993 are recommendations for testing based on the use of the final device. That said the lack of risk assessment in USP Class VI can be a problem. USP Class VI ISO 10993-5 Cytotoxicity In-Vitro Features Benefi ts.
Both iso 10993 and usp class vi define testing requirements for biocompatibility the ability of a material to perform a desired function without causing adverse effects on the human body. Unlike other rubber standards theres no one standard that engineers use for an approval. Usp class vi and iso 10993.
USP Class VI vs. Other Medical Device Regulations World-Wide. So does ISO 10993.
This post will take a deeper look at what biocompatibility is and how it is defined by the International Standards Organization. This standard uses a risk-based approach. A number of our plastic materials are ISO-10993 or USP Class VI capable.
This standard also utilizes systemic. In 1995 the FDA adopted ISO 10993 as its biocompatibility approach. ISO 134852016 - Medical Device Quality Management Systems.
While some of our rubbers can achieve this it is important to understand the customers explicit requirements. Food Grade or USP Class IV Materials for Manufacturing Injectable Products. Take an ASTM D2000 call out.
USP Class I II - Raw material supplier liability and responsibility. Usp class vi vs iso 10993. AAMI ISO 10993 2 2006 R 2014 Techstreet.
3D printing of dental and orthopedic surgical guides. Though not a limited series of tests some biocompatibility requirements for medical devices may exceed the testing performed in USP Class VI. Pick em predictions cs go 2019.

Regulatory Guidelines For Biocompatibility Safety Testing Mddionline Com

Material Selection Medical Injection Molding Xcentric Mold

Usp Class Vi Foster Corporation
Usp31nf26s1 C1031 General Chapters 1031 The Biocompatibility Of Materials Used In Drug Containers Medical Devices And Implants

Iso 10993 Vs Usp Class Vi Medical Molding And Bicompatible Rubber The Rubber Group

Understanding Food Grade Vs Biocompatibility For Medical Device Materials Medical Product Outsourcing

Pre Colored Medical Abs Compounds For Laser Marking Plastics Technology
Adhesives Protective Materials And Equipment For Medical Device Assembly Intertronics

Medical Grade Cyanoacrylate Super Glue Iso 10993 And Usp Class Vi

Understanding Food Grade Vs Biocompatibility For Medical Device Materials Medical Product Outsourcing
Material Selection Medical Injection Molding Xcentric Mold
Brilliant Mind The World Of Tubing For Medical Use Medical Plastics News
Usp31nf26s1 C1031 General Chapters 1031 The Biocompatibility Of Materials Used In Drug Containers Medical Devices And Implants

Advantapure Apst Silicone Tubing Translucent Platinum Cured
Usp31nf26s1 C1031 General Chapters 1031 The Biocompatibility Of Materials Used In Drug Containers Medical Devices And Implants

Regulatory Guidelines For Biocompatibility Safety Testing Mddionline Com

Looking Beyond Usp Class Vi Testing What Is Usp 87 Testing Holland Applied Technologies

Regulatory Guidelines For Biocompatibility Safety Testing Mddionline Com

